No CrossRef data available.
Article contents
Modifying chemical composition of the fine Ni4Nb2O9 powders using chloride melts as reaction medium
Published online by Cambridge University Press: 20 September 2019
Abstract
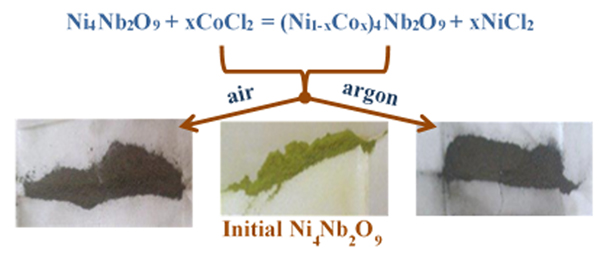
Thermal stable chloride melts were used as the reaction medium for modifying the chemical composition of complex oxides ensuring a marked improvement of their working properties. This paper discusses the original results of the direct effect of molten KCl–CoCl2 mixtures on the fine Ni4Nb2O9 powders under argon- and oxygen-containing gaseous atmospheres at 500 °C. The initial Ni4Nb2O9 powder and the reaction products were studied in detail using the differential scanning calorimetry, thermogravimetry, x-ray diffractometry, Raman and IR spectroscopies, scanning electron microscopy, energy-dispersive x-ray spectroscopy, chemical analysis, and conductometry which demonstrated clearly the formation of the thermal stable single-phase Ni–Co niobates.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2019



