No CrossRef data available.
Article contents
Effect of adjunctive aripiprazole in achieving various levels of response and on domains of functioning in MDD: A pooled analysis
Published online by Cambridge University Press: 16 April 2020
Abstract
Major Depressive Disorder (MDD) patients experience different levels of response and functionality impairments.
Evaluate levels of response for adjunctive aripiprazole therapy (AA) and effects of AA on patient functioning.
Data were pooled from three similar, randomized, double-blind, placebo-controlled trials with aripiprazole in MDD. Quartile response categories were defined by reduction (%) in MADRS >6 weeks of treatment: Minimal response (≤25%), Partial response (>25% to < 50%), Moderate response (≥50% to < 75%), and Robust response (≥75%). Proportions of placebo (AP) vs. AA patients achieving a response were compared (Cochran-Mantel-Haenszel test) for each category. Functionality was assessed using mean changes in Sheehan Disability Scale (SDS) scores. Changes in scores were compared (ANCOVA) between AA and AP.
AA had more patients (%) compared with AP achieving partial (23.9% vs. 17.9%, p = 0.017), moderate (23.1% vs. 15.0%, p< 0.001), and robust responses (14.3% vs. 7.4%, p< 0.001). AA had less (%) achieving minimal response compared with AP (38.7% vs. 59.6%, p< 0.001). Mean changes are below.
[Mean changes in SDS and domain scores]
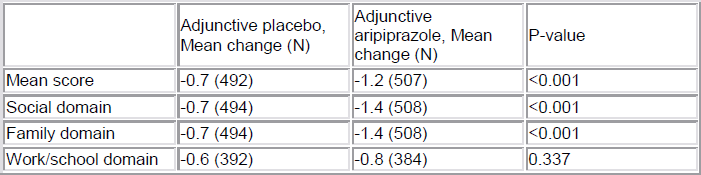
Most inadequate responders who continued on AP were minimal responders (60%). 60% of aripiprazole patients rapidly achieved partial, moderate or robust response status. AA significantly improved social and family life domains of functioning.
- Type
- P02-35
- Information
- European Psychiatry , Volume 26 , Issue S2: Abstracts of the 19th European Congress of Psychiatry , March 2011 , pp. 630
- Copyright
- Copyright © European Psychiatric Association 2011



Comments
No Comments have been published for this article.